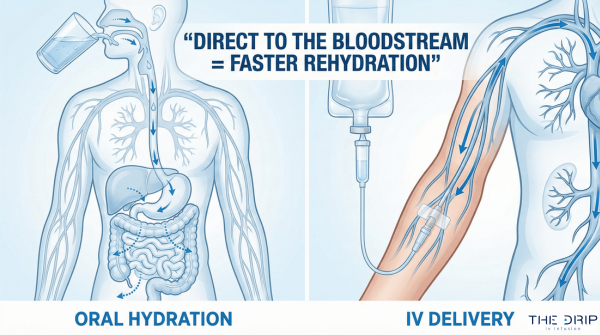
Regular intake of fluids and nutrients is important to maintain homeostasis in the body and establish balance. With the occurrence of injuries or diseases, this homeostasis can be disturbed, reducing the function of organs and metabolic processes. As a solution to this problem, many health professionals opt for crystalloids or colloids. So, what is the difference between crystalloid vs colloid?
In this article we will look at the main differences between these two solutions and their use for establishing normal bodily homeostasis.
Source: shutterstock.com / Photo Contributor: New Africa
Crystalloid vs Colloid
Fluids for infusion can be divided into two categories – colloids and crystalloids. Crystalloids are plasma volume expanders containing electrolytes such as potassium and sodium. Such crystals are able to dissolve in a solution and enable such a solution to move across membranes. Crystalloids are usually used to increase the intravascular volume when it is reduced.
Colloids, on the other hand, are gelatinous solutions that act as plasma volume expanders. Unlike crystalloids, the particles of colloids are too large to pass through semi-permeable membranes such as capillary membranes. Therefore, colloids remain in the intravascular space longer than crystalloids. An example of colloids are dextran, hydroxyethyl starch, albumin, and Haemaccel.
It’s worth noting that both solutions are administered in the IV hydration Arizona.
Crystalloid solutions
Medical professionals widely use crystalloids to maintain homeostasis and replace lost electrolytes. Such solutions are composed of water and electrolytes, which makes them suitable for improving the electrolyte and fluid imbalance in the body. The most common crystalloids used for IV hydration are:
Sodium chloride
Normal saline solution (0.9% sodium chloride), or physiological saline solution, is the most common crystalloid used in clinical practice. Due to its composition, it has a similar tonicity to the extracellular fluid.
Normal saline is commonly used for drug administration, fluid resuscitation, and intravascular volume expansion.
Ringer’s lactate solution
Ringer’s lactate (RL) solution is a mixture of sodium chloride, calcium chloride, potassium chloride, and sodium lactate in water. This mixture is usually administered in case of surgery, dehydration, or receiving medications through IV. In addition, this solution can be used in mild metabolic acidosis because it has the ability to metabolize lactate in the liver.
Hartmann’s solution
Hartmann’s solution (Compound Sodium Lactate) is similar to lactated Ringer’s solution except that it contains sodium bicarbonate in its composition. In addition, this solution is isotonic and provides electrolytes that have concentrations similar to the extracellular fluid.
When it comes to use, Hartmann’s solution is used for fluid resuscitation, especially in cases of hypovolemia and dehydration, as well as perioperative fluid management.
Hypertonic saline
Unlike normal saline, which may contain 3% or 5% sodium chloride, hypertonic solutions contain a higher concentration of sodium chloride. Regarding its use, such solutions have specific uses in cases such as hyponatremia, hypovolemic shock, and treating cerebral edema.
In addition, hypertonic solutions are characterized by an osmotic effect. With this property, these solutions draw water from the interstitial and intracellular compartments into the intravascular space, thus increasing the intravascular volume by improving perfusion.
Colloid solutions
Colloid solutions are used to replace lost fluids to increase intravascular volume by increasing oncotic pressure in the bloodstream.
Unlike crystalloids, colloids contain larger molecules suspended in a liquid base, allowing fluid to be retained in blood vessels. In the following, we will look at some of the colloidal solutions that are most often used in medical practice:
Albumin solutions
Albumin solutions are one of the most used colloidal solutions in clinical practice. Namely, albumin is a natural protein found in plasma that increases intravascular oncotic pressure.
Such solutions are usually available in various concentrations, such as 5% and 25%. They are used in patients with hypoalbuminemia, hypovolemia and in those conditions where expansion of the plasma volume is required.
Hydroxyethyl starch
Hydroxyethyl starch is a synthetic starch commonly used for fluid resuscitation to replace intravascular pressure. This colloidal solution has different molecular weights and concentrations, affecting pharmacodynamic and pharmacokinetic properties.
When it comes to its use, hydroxyethyl starch is most commonly used for hypovolemic shock and critically ill patients.
Gelatin solution
Gelatin-based colloidal solutions are composed of hydrolyzed gelatin derived from animal sources. Such solutions are used for volume replacement in those situations requiring rapid intravascular expansion, usually in surgery or trauma.
Although such solutions are well tolerated, caution should be exercised in their application in persons with a history of sensitivity to gelatin.
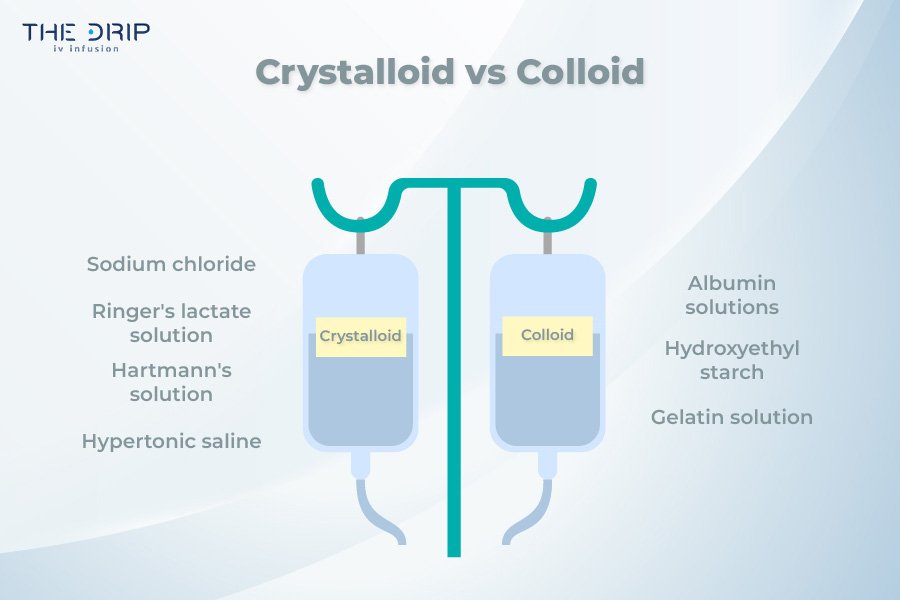
Differences Between Crystalloid and Colloid Solutions
When choosing a solution to replenish lost fluids, one should know what distinguishes crystalloids and colloids. So, what is the difference between a colloid and a crystalloid?
Molecular size and properties – Crystalloids comprise small molecules such as ions and small solutes, while colloids consist of larger molecules suspended in solution.
Volume expansion capabilities – Crystalloid solutions can effectively expand intravascular volume, and their effect is short-lived. On the other hand, colloids expand the volume by increasing the intravascular volume and have a longer effect.
Duration of intravascular retention – Crystalloids have a relatively short duration of intravascular retention in contrast to colloids, which in turn have prolonged retention due to the size of the molecules.
Hemodynamic effects – Crystalloids expand plasma volume by replenishing fluid deficits. On the other hand, colloids increase intravascular volume by increasing oncotic pressure.
Potential adverse effects and complications – When it comes to adverse effects, crystalloids are generally safe to use. But when crystalloids are administered in large volumes, it may lead to electrolyte imbalance, tissue edema or dilutional coagulopathy. On the other hand, the negative effects of colloid solutions may include allergic reactions, tissue edema, and coagulopathy.
Ready for Better Hydration with IV Therapy ?
Professional hydration therapy ensures that the right fluids are administered safely and effectively. For individuals seeking convenient, on-demand care, mobile IV therapy brings hydration and recovery directly to their location in Gilbert.
Can You Get Mobile IV Therapy in Gilbert for Hydration and Recovery?
Yes, you can get mobile IV therapy in Gilbert for hydration and recovery, making treatment convenient and personalized. Mobile IV therapy in Gilbert delivers fluids directly to your home, workplace, or event, helping restore balance with the right mix of crystalloid and colloid solutions when needed. Whether you need rapid hydration, recovery after illness, or support for conditions like dehydration and fatigue, our licensed professionals ensure safe and effective care. Learn more about [Mobile IV Therapy in Gilbert] to see how it can support your wellness and recovery.
Is Understanding Crystalloids vs Colloids Essential for IV Therapy?
Yes, understanding crystalloids vs colloids is essential for IV therapy because it helps patients and providers choose the most effective treatment. By knowing the differences in fluid retention, electrolyte balance, and plasma volume expansion, you can better appreciate how IV therapy supports hydration and recovery. When comparing these two solutions, remember there is no one-size-fits-all solution for all patients. Both solutions come with their advantages, and the choice depends on several factors, such as the patient’s health condition, clinical scenario, and available resources.
However, when administering such solutions, healthcare professionals should consider the patient’s individual needs as well as the potential risks that may arise during their administration.