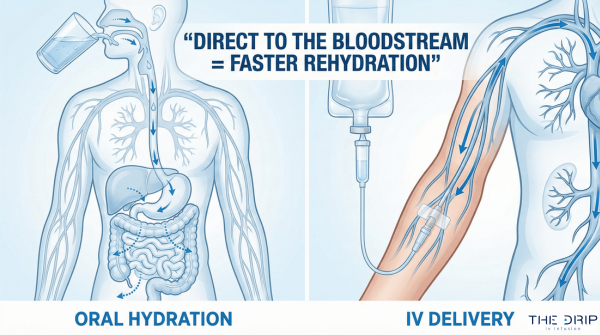
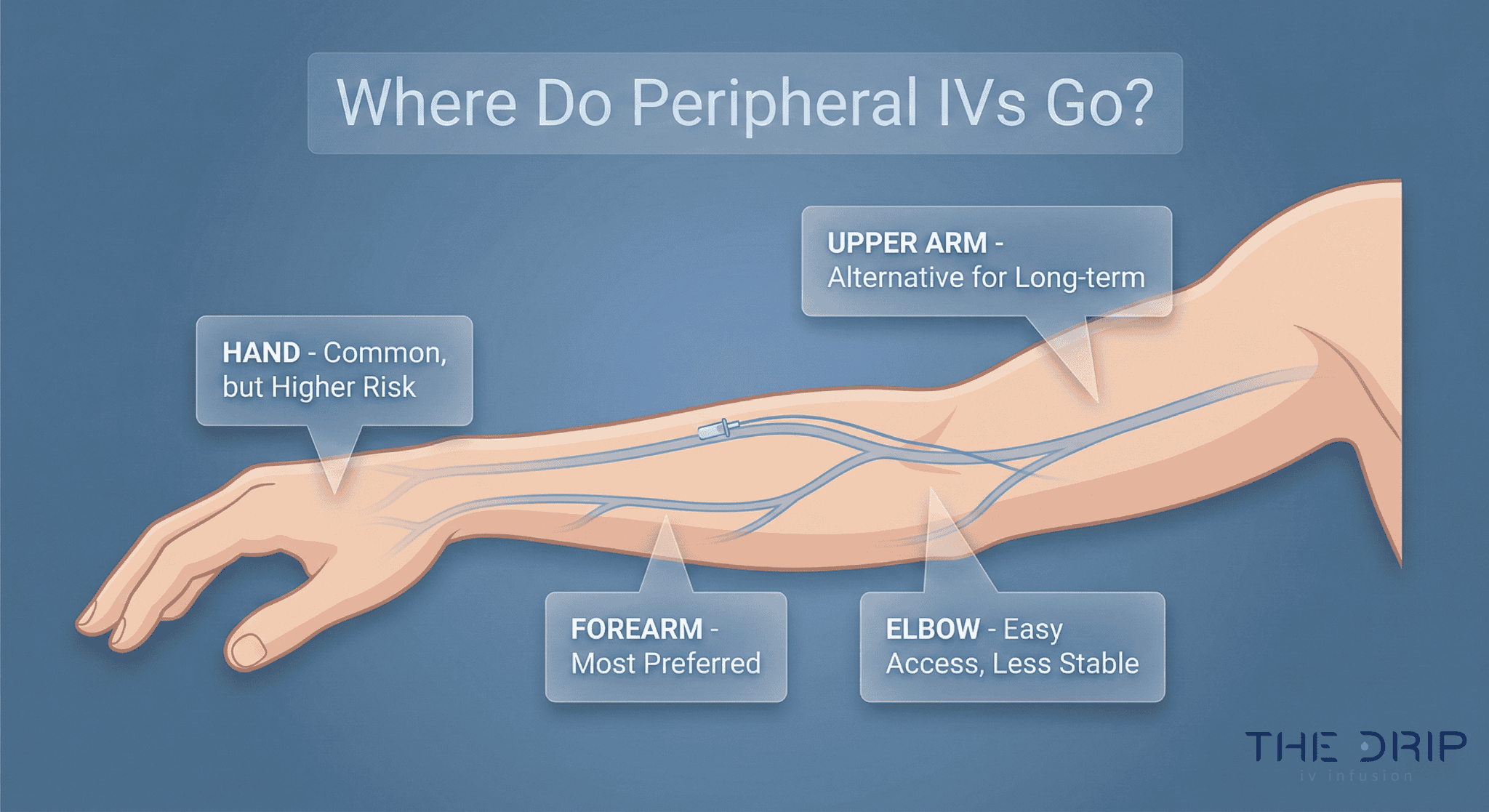
Intravenous therapy has gained a lot of popularity among Americans. It is used by people seeking relief from certain health and wellness difficulties. Hence, the government has passed several IV therapy regulations to answer the increasing demand for the services.
Before you get an IV therapy treatment, it is important to check the laws for administering IV fluids outside of a hospital setting. The complex set of regulations obligates every service provider to prioritize safety, quality, and ethical practices.

Source: shutterstock.com / Photo Contributor: Song_about_summer
IV Therapy Regulations
IV therapy has wide use, from hydration and vitamin supplementation to morning sickness relief and weight loss support. Modern IV therapy has been used for almost a century, but there is a lack of federal laws in the US for such treatments.
Federal regulations of IV therapy
According to federal law, IV therapy is a compound drug because it is a combination of drug ingredients. IV therapy on a federal level is regulated by Section 503A of the Federal Food, Drug, and Cosmetic Act.
The federal IV therapy regulations also include requirements for preparing and administering IV treatments. Every IV therapy business should meet Pharmaceutical compounding and Sterile Preparations standards. So, mobile IV clinics should ensure they meet the following regulations:
A licensed pharmacist or a licensed physician must handle the IV therapy compounding.
All bulk drug substances must have a valid certificate of analysis and be manufactured by an FDA-registered establishment.
The compounding personnel must be supervised and properly trained.
The facility must have sanitary conditions for drug compound preparation.
State regulations of IV therapy
The little intervention of the federal government in the industry has led state governments to pass laws for IV therapy administration. One of the most important is the Corporate Practice of Medicine. The regulation concentrates on people who can practice medicine.
Unfortunately, not every state has a CPOM. Those 33 states have CPOM ranging from only basic regulations to rigorous enforcement. Every state has its unique regulations. Most of them differ regarding two questions:
Who can launch and operate the medical corporation?
Who is allowed to administer the fluids?
Ownership and licenses
The regulations from one state to another might vary. For instance, some states, like Florida, don’t require a license to launch an IV therapy business. Other states require obtaining licenses and permits from local, state, or federal governments.
On the other hand, in states like California, you must have a medical corporation with 51% ownership from a licensed physician and 49% ownership can be from allied health professionals.

Source: shutterstock.com / Photo Contributor: Dmytro Zinkevych
People allowed to administer IV therapy
Before administering the IV solution, the people administering it must ensure the patient will receive the appropriate care according to their symptoms, diagnosis, and treatment needs.
An evaluation exam should determine whether the patient is a suitable candidate for IV therapy. Additionally, the staff is responsible for filling out the needed documentation. Moreover, the service provider should keep it according to the law requirements. In most states, people who are allowed to administer IV therapy are:
Physician assistants
Nurse practitioners
Registered mobile infusion nurses
Licensed practical nurses
Licensed vocational nurses
However, the state regulations also differ on this subject. To illustrate, there are states in the USA, like California, where additional supervision is required in certain cases.
More specifically, registered nurses are only allowed to administer IV infusions under supervision. The supervisor can be a nurse practitioner, physician, or physician assistant.
No matter the medical title, certification, and licensing are a must. Only people with nursing board-approved intravenous therapy courses or equivalent certifications in the USA can administer IV therapy.
Challenges to IV Therapy Regulation
The IV therapy regulations face many notable issues in the USA. The first one is the lack of standardization across states. Without unified laws on a federal level, there is always the risk of inconsistencies in medical practice. As a consequence, patient care can be compromised.
Furthermore, rigorous standards are needed to avoid exposing patients to unqualified practitioners. The IV therapy regulations should specifically list the scope of work for IV therapy businesses. With such regulations, potential misuse and treatment abuse might be avoided.
The regulations for IV therapy should also regulate who can make the IV bags. There should be clear laws about calculating the proper dosages. Additionally, there is a need for more rigorous regulations about the ingredient’s origin and IV bag preparation.
Future of IV Therapy Regulation
IV therapy has overtaken the medical world. Healthcare professionals will likely continue collaborating and advancing the IV therapy treatment options. With the potential developments in technology and medical findings, IV therapy might grow to target more everyday problems people face.
Considering the challenges in the industry, it is likely that states will push toward greater standardization and treatment oversight. A possible change is a uniform and comprehensive approach to IV therapy practices on a federal level.
All things considered, the regulatory bodies must continue collecting data and researching the market growth. Every change in the sector and treatment option must be closely monitored to ensure patients get adequate and safe treatments.
Many believe that IV therapy providers should be categorized as healthcare service providers. For better stability and sustainability of the industry, clinical policies and procedures should be implemented.
Altogether, the expectations are that once the industry is more regulated, the number of IV therapy administered in non-traditional settings will continue to grow.

Source: shutterstock.com / Photo Contributor: Studio Romantic
Conclusion
Everyone interested in getting intravenous treatment must ensure the service provider complies with the IV therapy regulations. Also, companies offering IV solutions must ensure they follow the rules to maximize the safety and quality of the treatments while working according to ethical principles.