If you’re struggling with arthritis pain and seeking effective treatment options, you’ve likely wondered about intravenous (IV) therapies that deliver medications directly into your bloodstream. We understand the frustration of managing chronic joint pain and the search for treatments that can provide meaningful relief. You’re in the right place to learn about how IV therapy could potentially help manage your arthritis symptoms more effectively than traditional oral medications.
IV therapy for arthritis is a medical treatment that delivers disease-modifying antirheumatic drugs (DMARDs), biologics, or nutrients directly into the bloodstream through an intravenous infusion, bypassing the digestive system to achieve 100% bioavailability and potentially faster, more effective symptom relief compared to oral medications. According to a 2025 network meta-analysis by Liu et al., evaluating 86 randomized controlled trials with data on rheumatoid arthritis patients, infliximab combined with methotrexate demonstrated the highest efficacy ranking with an odds ratio of 10.53 for achieving ACR50 response rates. Dr. Jorge Mamasaidov from the 2025 systematic review on quality of life improvements states, “The findings suggest that bDMARDs significantly improve quality of life in RA patients by reducing pain, fatigue, and disability.”
Key Takeaways:
• IV medications achieve 100% bioavailability compared to approximately 73% for oral medications, allowing faster symptom relief
• TNF-α inhibitors and IL-6 receptor antibodies work by blocking specific inflammatory pathways in arthritis
• Both rheumatoid arthritis and psoriatic arthritis patients show significant improvements with IV biologics
• Common IV treatments include infliximab, rituximab, tocilizumab, and abatacept, each with specific dosing schedules
• Potential benefits include reduced pain, improved physical function, and better quality of life scores
• Important risks include increased infection risk and the need for regular monitoring
• Eligibility depends on previous treatment response and absence of contraindications like active infections
• IV therapy often complements traditional treatments and may be covered by insurance with prior authorization
This comprehensive guide explores how IV therapy addresses arthritis at the molecular level, examining the mechanisms that make these treatments effective for different types of inflammatory arthritis. We’ll review the scientific evidence supporting various IV medications, discuss who makes an ideal candidate for this treatment approach, and compare IV therapy to other arthritis management options. Throughout this article, we’ll provide insights into what patients can expect during treatment sessions, how to find qualified providers, and important safety considerations to help you make informed decisions about your arthritis care.
Practical Tip: Before considering IV therapy for arthritis, maintain a detailed symptom diary for at least 2-4 weeks documenting your pain levels, joint stiffness, and how symptoms affect daily activities—this information will help your rheumatologist determine if you’re an appropriate candidate for IV biologics and establish a baseline for measuring treatment effectiveness.
As we delve into the specifics of IV therapy for arthritis, we’ll equip you with the knowledge needed to have informed discussions with your healthcare team about whether this treatment approach aligns with your health goals and circumstances.
How Does IV Therapy Work to Address Arthritis Symptoms?
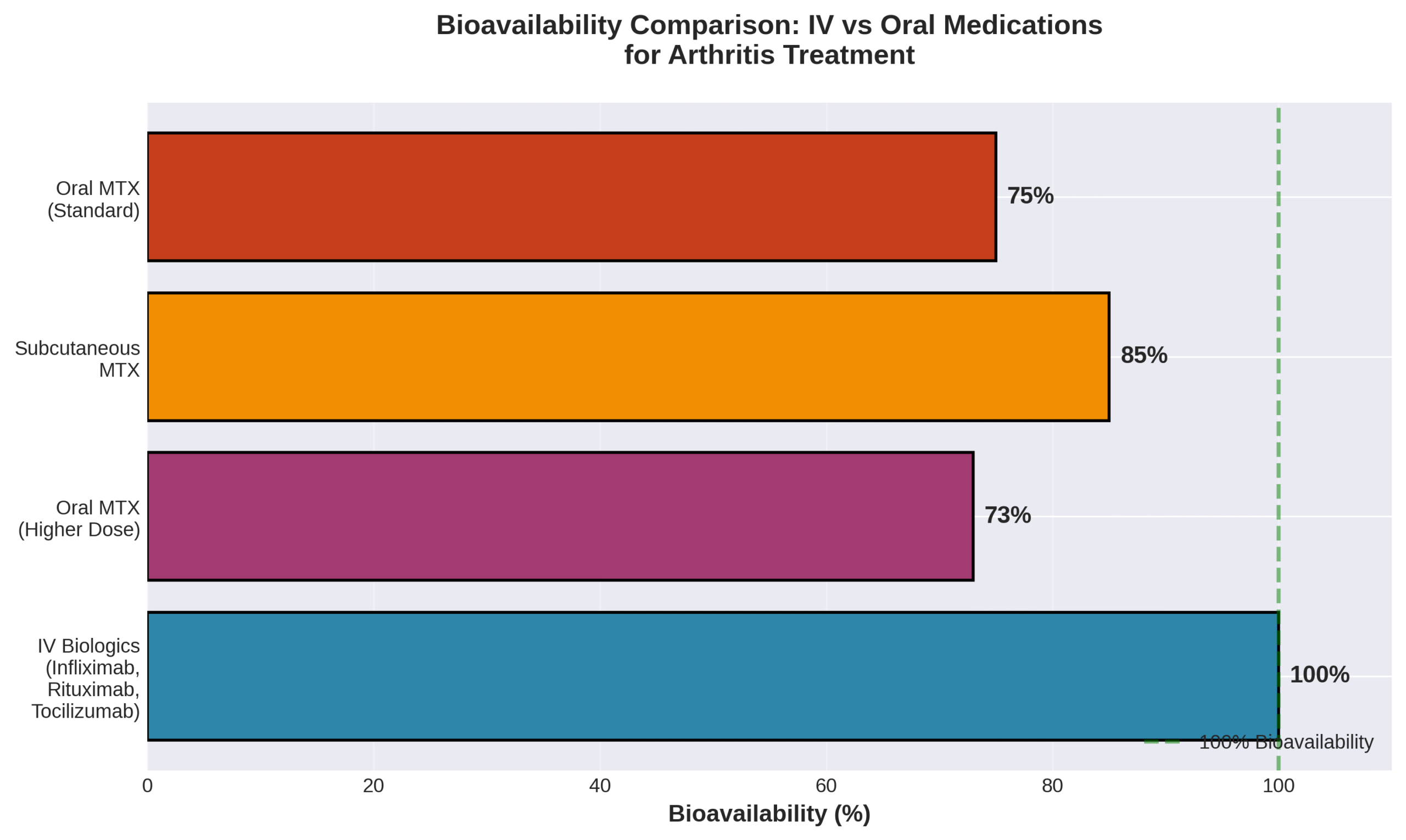
IV therapy for arthritis works by delivering medications directly into the bloodstream, achieving 100% bioavailability compared to 73% for oral medications at higher doses. This direct delivery method allows arthritis medications to bypass digestive system absorption barriers and reach target tissues faster. The most effective mechanisms involve blocking specific inflammatory proteins and depleting immune cells that drive joint inflammation. Understanding these mechanisms and the specific medications used helps patients make informed treatment decisions.
What Mechanisms Make IV Therapy Effective for Arthritis?
The mechanisms that make IV therapy effective for arthritis center on targeted immune system modulation through direct bloodstream delivery. TNF-α inhibitors such as infliximab, adalimumab, etanercept, and golimumab reduce inflammation by blocking TNF-α from binding to its receptors TNFR1 or TNFR2. IL-6 receptor antibodies including tocilizumab and sarilumab bind to the D3 domain of IL-6R, ameliorating symptoms and normalizing acute-phase response.
Rituximab works through a different mechanism by inducing almost complete depletion of peripheral blood B cell populations. This depletion typically lasts 6 to 9 months and associates with clinical response, leading to long-term therapy maintenance. The 100% bioavailability of IV medications ensures complete drug absorption, allowing patients to experience symptom relief earlier than with oral alternatives.
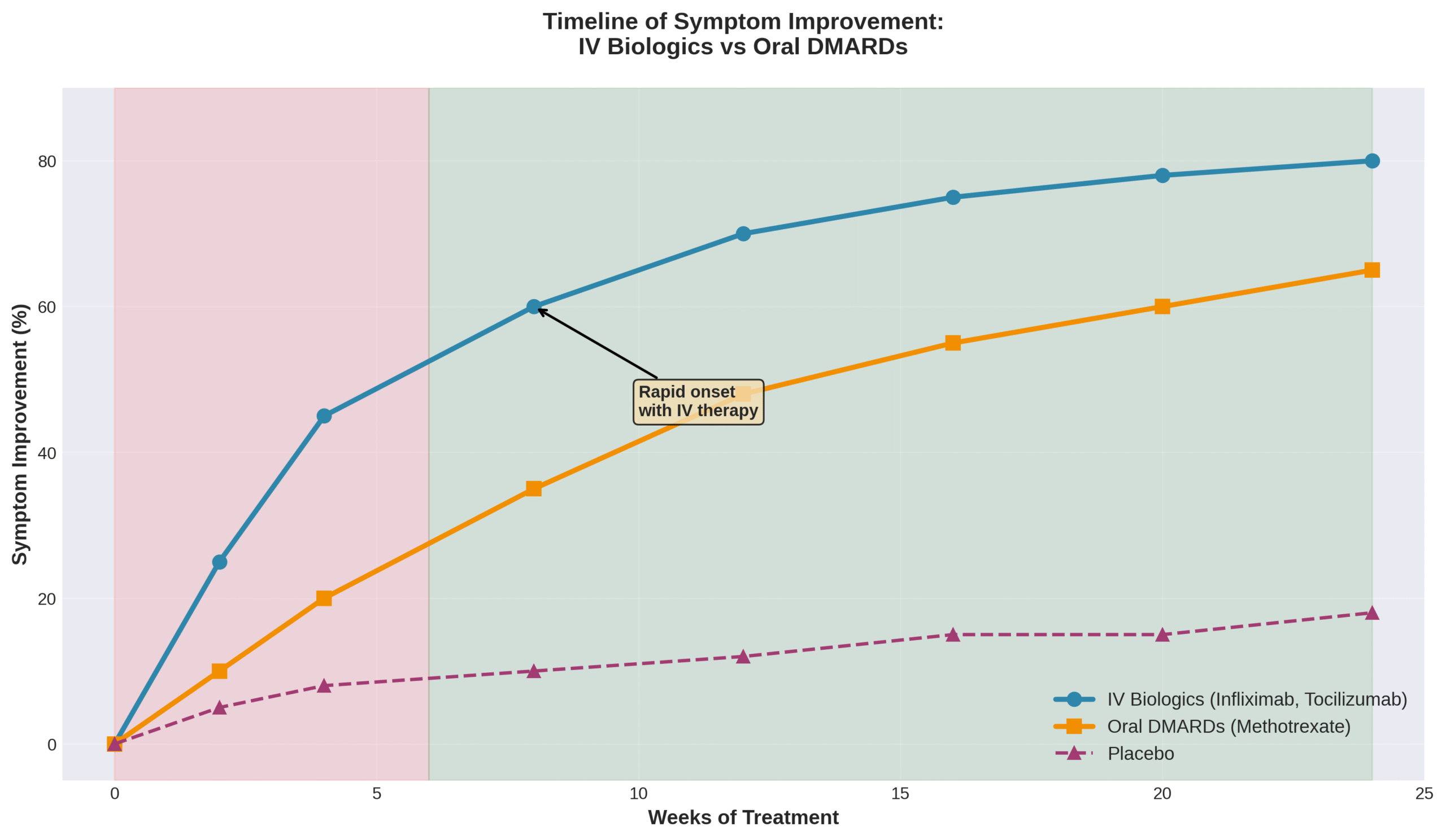
These targeted mechanisms work at the molecular level to interrupt inflammatory cascades that damage joints. The direct IV delivery ensures consistent therapeutic levels that oral medications cannot match due to variable absorption rates.
Which Nutrients or Medications Are Commonly Used in IV Therapy for Arthritis?
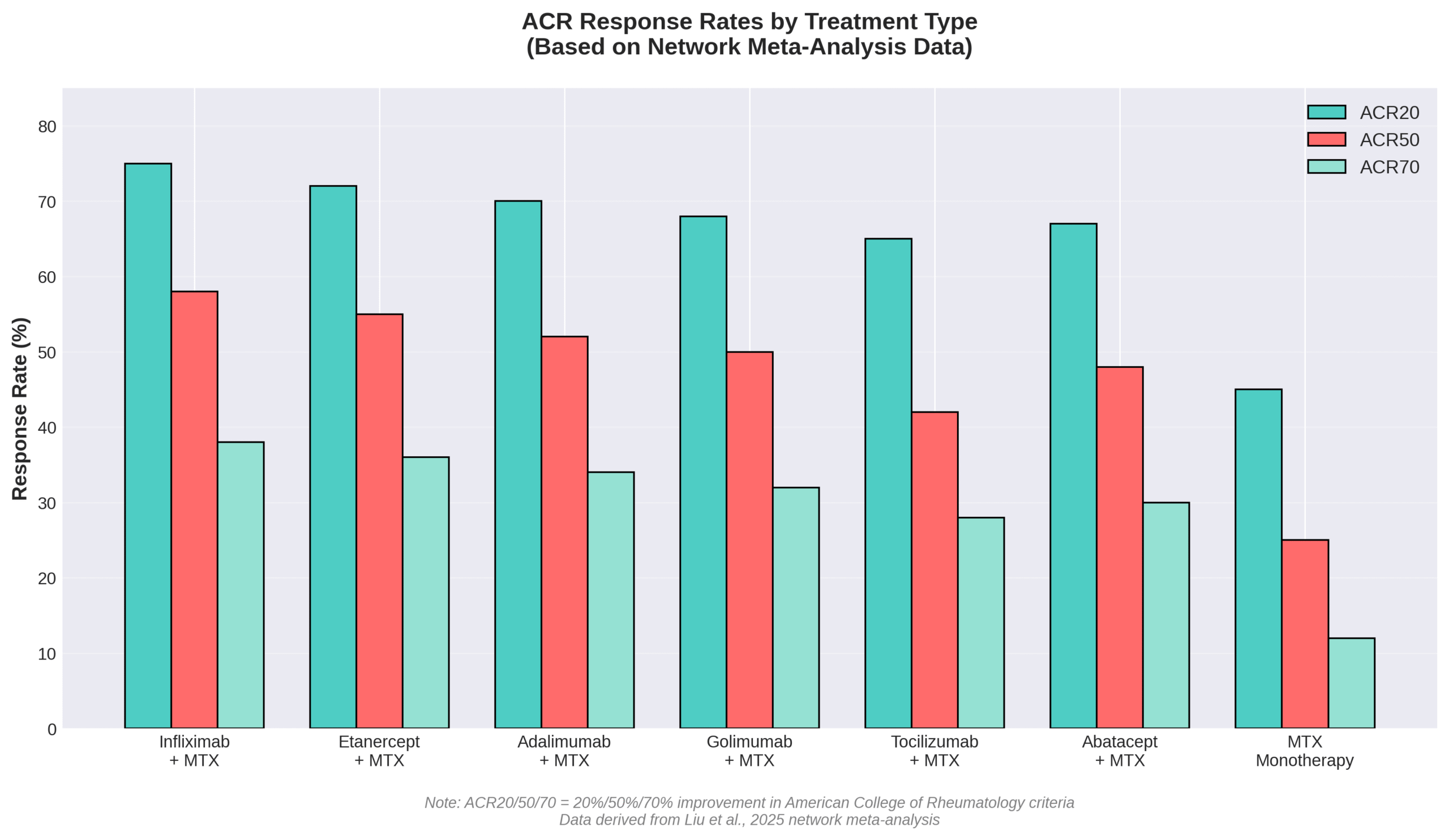
The nutrients and medications commonly used in IV therapy for arthritis include biologics with specific dosing protocols and proven efficacy rankings. A network meta-analysis showed infliximab combined with methotrexate had the highest efficacy ranking with an odds ratio of 10.53 and SUCRA score of 0.884. Tocilizumab administers as a 60-minute single intravenous drip infusion with doses not exceeding 800 mg per infusion.
| Medication | Dosing Protocol | Key Features |
| Rituximab | 1000 mg IV over 3-4 hours, two doses 2 weeks apart | B-cell depletion for 6-9 months |
| Abatacept | Day 1, 15, 29, then every 4 weeks | 4-week maintenance schedule |
| Golimumab-IV | Weight-based dosing | Lower infusion reaction rates vs infliximab |
| Etanercept + MTX | Combined therapy | Highest safety ranking (OR = 0.29, SUCRA: 0.893) |
These medications target different inflammatory pathways, allowing personalized treatment based on patient response and tolerability. The combination therapies often show superior outcomes compared to monotherapy approaches. Each medication follows strict administration protocols to maximize effectiveness while minimizing adverse reactions.
What Types of Arthritis May Benefit from IV Therapy?
IV therapy benefits multiple arthritis types through targeted biologic medications that reduce inflammation and slow disease progression. The types of arthritis that benefit from IV therapy include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, each responding differently to treatment protocols.
How Does IV Therapy Differ for Rheumatoid vs. Osteoarthritis?
IV therapy for rheumatoid arthritis differs from osteoarthritis treatment because rheumatoid arthritis responds to disease-modifying antirheumatic drugs while osteoarthritis lacks approved IV biologics. A network meta-analysis of 86 randomized controlled trials showed most DMARDs combined with MTX achieved ACR50 significantly better than MTX monotherapy (p < 0.05) for RA. Real-world studies evaluating 182,098 RA patients demonstrated effectiveness of various biologics with primary non-response rates at 4.25 per 100 patient-years.
Rheumatoid arthritis patients experience greater physical health improvements than mental health benefits from biologic agents. The FDA approved new drugs for rheumatoid arthritis treatment throughout 2024, expanding IV therapy options. Osteoarthritis patients lack access to these IV biologics since osteoarthritis involves mechanical joint damage rather than autoimmune inflammation.
These differences guide treatment selection between the two arthritis types, preparing patients for distinct therapeutic approaches.
Are Other Forms of Inflammatory Arthritis Suitable for IV Therapy?
Other inflammatory arthritis forms suitable for IV therapy are psoriatic arthritis, ankylosing spondylitis, and giant cell arteritis. A study of 12,262 biologic-naïve PsA patients showed 6 months of TNFi treatment reduced pain by approximately 50%. Biologic therapy produces short- and long-term improvements in disease activity and health-related quality of life for both psoriatic arthritis and ankylosing spondylitis patients.
Patient satisfaction remains high across these conditions, with AS, PsA, and RA patients expressing strong satisfaction with IV biologic medications and overall treatment. Biosimilars received approval in IV formulations for treating RA, giant cell arteritis, and polyarticular conditions, expanding access to treatment. MRI imaging documented measurable inflammation reduction in psoriatic patients, with scores decreasing from 9 to 6 on a 0 to 36 scale following biologic treatment.
Understanding which arthritis types respond to IV therapy helps patients and providers determine appropriate treatment pathways for optimal symptom management.
What Are the Potential Benefits and Risks of IV Therapy for Arthritis?
The potential benefits and risks of IV therapy for arthritis involve significant improvements in quality of life balanced against infection risks and other complications. Biologic DMARDs administered intravenously reduce pain, fatigue, and disability while requiring careful monitoring for adverse events.
What Improvements Do Patients Often Report with IV Therapy?
Patients often report improvements in pain reduction, physical function, and overall quality of life with IV therapy. According to a 2021 study on biologic-naive RA patients, bDMARDs significantly improved HAQ-DI scores, SF-36 physical and mental component scores, EQ-5D-3L index, and work productivity measures. Adalimumab treatment demonstrated greater changes in Health Assessment Questionnaire Disability Index scores, directly improving daily life performance.
IV golimumab and infliximab achieve comparable improvements across PROMIS measures for social, mental, and physical well-being. A 2020 clinical trial found selective B-cell depletion with rituximab led to sustained clinical improvements in RA patients. Survey data from multiple healthcare systems indicate high satisfaction levels with IV biologic administration among arthritis patients.
These improvements translate to enhanced mobility, reduced morning stiffness, and better work attendance. The upcoming section details potential complications requiring medical attention.
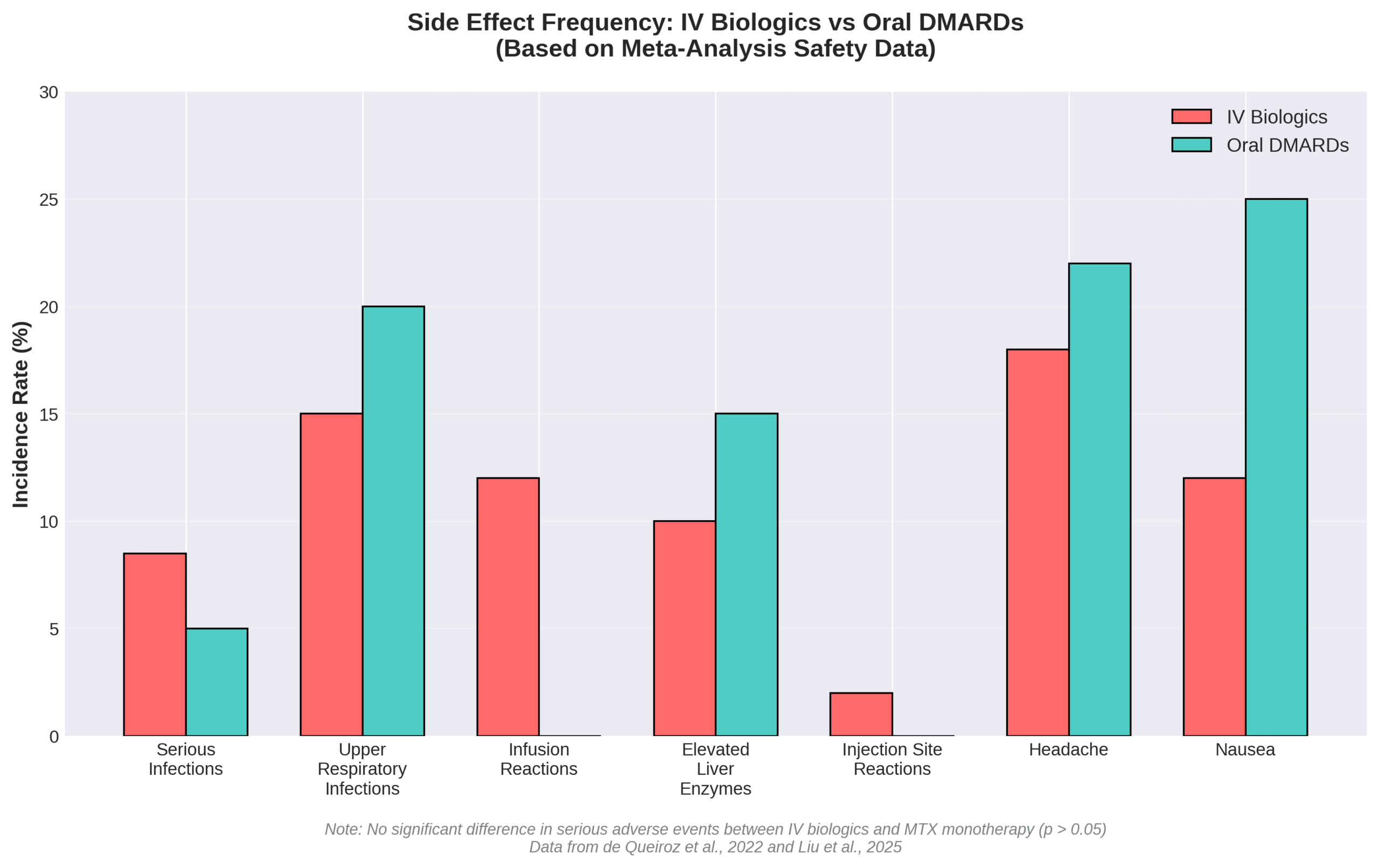
What Side Effects or Complications Should Patients Be Aware Of?
Patients should be aware of serious infections, hepatic injury, and injection site reactions as primary complications. The FDA issued a Boxed Warning for tocilizumab regarding serious infections leading to hospitalization or death. Common serious infections include pneumonia, urinary tract infection, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis, and bacterial infections.
All biologic DMARDs increase infection risk across bacterial, fungal, and viral categories. A 2022 safety analysis found tocilizumab caused serious hepatic injury cases, with some requiring liver transplantation. Common adverse reactions encompass upper respiratory tract infections, elevated liver enzymes, and injection site reactions.
| Complication Type | Specific Risk | Severity |
| Infections | Pneumonia, UTI, cellulitis | Hospitalization possible |
| Hepatic | Liver enzyme elevation | Transplant in rare cases |
| Local reactions | Injection site pain | Mild to moderate |
| Immune | Herpes zoster reactivation | Requires antiviral treatment |
Despite these risks, clinical trials show serious adverse event incidence was not significantly different from methotrexate monotherapy (p > 0.05). Careful patient selection and monitoring protocols help minimize complications while maximizing therapeutic benefits for arthritis management.
Who Is an Ideal Candidate for IV Therapy for Arthritis?
IV therapy for arthritis is reserved for patients with specific inflammatory conditions who meet strict medical criteria. The American College of Rheumatology (ACR) recommends biologics for patients who have not responded adequately to conventional disease-modifying antirheumatic drugs (DMARDs). Healthcare providers evaluate each patient’s medical history, current medications, and comorbidities before determining eligibility. The following sections outline exclusion criteria and eligibility determination processes.
Are There Any Medical Conditions That Exclude Someone from IV Therapy?
Medical conditions that exclude someone from IV therapy include severe hypersensitivity reactions to specific medications and active infections. Severe hypersensitivity to infliximab or its inactive ingredients contraindicates treatment. Known hypersensitivity to tocilizumab products is a contraindication. Severe infections contraindicate infliximab treatment due to immunosuppression risks.
The ACR 2021 guidelines address use in high-risk populations such as those with:
- Liver disease
- Heart failure
- Lymphoproliferative disorders
- Serious infections
NSAIDs remain generally safe when used in combination with DMARDs. Patients with these conditions require careful evaluation and potential alternative treatments.
How Is Eligibility Determined by Healthcare Professionals?
Healthcare professionals determine eligibility through systematic evaluation using ACR guidelines and shared decision-making. Methotrexate remains the recommended first choice over biologics for most people with inflammatory autoimmune diseases. The ACR provides 44 recommendations for rheumatoid arthritis treatment, including 7 strong and 37 conditional recommendations based on GRADE methodology.
Most complex infusion therapies, including IVIG and biologics, require prior authorization from insurance providers. Individual treatment decisions incorporate patients’ values, goals, preferences, and comorbidities through shared decision-making processes. This collaborative approach ensures treatment aligns with patient needs while following evidence-based protocols.
How Does IV Therapy Compare to Other Arthritis Treatments?
IV therapy delivers arthritis medications directly into the bloodstream, achieving 100% bioavailability compared to 73% for oral methotrexate at higher doses. This complete absorption allows faster symptom relief than pills or subcutaneous injections. The intravenous route bypasses digestive system limitations that reduce oral medication effectiveness. Understanding these differences helps patients and providers select optimal treatment approaches for arthritis management.
What Are the Advantages and Limitations Compared to Oral or Injectable Medications?
The advantages and limitations compared to oral or injectable medications center on bioavailability, effectiveness rates, and drug-specific performance metrics. IV medications achieve 100% bioavailability while oral methotrexate reaches only 73% absorption at higher doses. Subcutaneous methotrexate shows significantly higher bioavailability than oral forms at equivalent dosages.
Research comparing biologic effectiveness reveals important distinctions. A meta-analysis found TNF inhibitors showed lower effectiveness than non-TNF biologics (RR: 0.88; 95% CI: 0.81–0.95; p < 0.01). Biologic DMARDs demonstrated reduced effectiveness compared to JAK inhibitors (RR: 0.86; 95% CI: 0.79–0.94; p < 0.01). Among TNF inhibitors, adalimumab, etanercept, and golimumab outperformed infliximab (RR: 1.19; 95% CI: 1.05–1.36; p < 0.01).
| Medication Route | Bioavailability | Key Finding | Source Data |
| IV medications | 100% | Complete absorption | Definition |
| Oral MTX (high dose) | 73% | Reduced at higher doses | Clinical data |
| Subcutaneous MTX | >73% | Superior to oral | Comparative studies |
| TNF inhibitors | Variable | Lower than non-TNF biologics | RR: 0.88 |
These bioavailability differences translate into clinical practice considerations for arthritis treatment selection.
Can IV Therapy Be Used Alongside Traditional Arthritis Therapies?
IV therapy can be used alongside traditional arthritis therapies, with combination approaches often showing superior outcomes. Research shows no significant differences between biologic monotherapy and combination therapy effectiveness (RR: 0.83; 95% CI: 0.68–1.00; p < 0.01). Most DMARDs combined with methotrexate achieved ACR50 response significantly better than methotrexate monotherapy.
Infliximab follows a specific combination protocol: 3 mg/kg IV at weeks 0, 2, and 6, then every 8 weeks with concurrent methotrexate. NSAIDs such as aspirin, ibuprofen, and naproxen remain generally safe when combined with DMARDs. Treatment integration includes medications, joint stress reduction, physical therapy, occupational therapy, and surgical interventions when necessary.
The compatibility of IV biologics with traditional therapies enables comprehensive arthritis management strategies that address multiple disease aspects simultaneously.
What Should Patients Expect During an IV Therapy Session for Arthritis?
Patients receiving IV therapy for arthritis undergo structured infusion protocols with specific timing and monitoring requirements. The typical procedure involves arriving at an infusion center, undergoing vital sign checks, and receiving medications through controlled intravenous administration while medical staff monitor for reactions.
What Is the Typical Procedure and How Long Does It Take?
The typical procedure and how long it takes varies by medication type. Infliximab requires IV infusion for at least 2 hours to ensure safe delivery. Rituximab first infusion starts at 50 mg/hr and must not be administered as IV push or bolus to prevent severe reactions. Tocilizumab administration occurs as a 60-minute single intravenous drip infusion with careful monitoring throughout.
Abatacept follows a specific schedule with doses on Day 1, Day 15, and Day 29, then every 4 weeks thereafter. Each infusion session includes pre-medication protocols, vital sign monitoring, and post-infusion observation periods. IV infusion therapy provides direct and efficient medication delivery into the bloodstream, bypassing digestive system absorption issues.
Medical teams prepare patients by checking allergies, reviewing medications, and establishing IV access before starting infusions. Standard protocols require continuous monitoring during administration to detect any adverse reactions immediately.
How Many Sessions Are Usually Needed for Noticeable Results?
The number of sessions needed for noticeable results depends on the specific biologic medication prescribed. Infliximab requires 7 infusions per year, with induction doses at weeks 0, 2, and 6, followed by maintenance every 8 weeks. Rituximab involves 4 infusions annually, with retreatment every 6 months based on clinical evaluation but no sooner than every 4 months.
Treatment response timelines show that 6 months of TNFi treatment reduced pain by approximately 50% in PsA patients. Patient preferences reveal that 63.4% would choose an infusion every 6-9 months for RA therapy versus 21.5% preferring tablets only. Golimumab demonstrated benefits both before and after methotrexate therapy in radiographic outcomes.
Most patients experience initial improvements within the first 3-6 infusions, though individual responses vary based on disease severity and previous treatments. Understanding these timelines helps patients set realistic expectations for their arthritis management journey with IV therapy at The Drip IV Infusion.
How Do You Find a Qualified Provider for IV Therapy for Arthritis?
Finding the right provider for IV therapy requires verifying specific certifications and safety protocols. The provider selection process determines treatment safety and effectiveness for arthritis management.
What Credentials and Experience Should You Look For?
The credentials required for IV therapy providers include CRNI® (Certified Registered Nurse Infusion) certification for infusion nurses. Providers must maintain a current, active, unrestricted RN license in the US or their country of practice. Some states enforce specific guidelines for infusion therapy education, training, and competency validation.
Joint Commission certification participation requirements encompass Clinical Information Management, Collaboration, and Compounding preparations. Home infusion therapy demands specific accreditation and eligibility requirements per CMS regulations. These credentials ensure providers possess the specialized knowledge to administer complex IV medications safely.
Healthcare facilities offering IV therapy should display their accreditation certificates and staff credentials prominently. Qualified providers undergo continuous education to maintain their certifications and stay current with evolving treatment protocols.
How Can You Ensure Safety and Proper Protocols Are Followed?
Safety and proper protocols are ensured through multiple verification systems. Most complex infusion therapies require prior authorization processes that verify medical necessity and treatment appropriateness. Biologic infusions remain well-tolerated, safe, and effective in most patients when protocols are followed correctly.
Strong ACR recommendations require 70% level of agreement by voting panel members before implementation. Guidelines serve as frameworks rather than prescriptive rules, with individual decisions made through shared decision-making processes between providers and patients. Updated EULAR recommendations provide consensus on RA management including safety, effectiveness, and cost considerations.
Providers should document all safety protocols, maintain detailed treatment records, and conduct regular safety audits. Patients can request information about facility safety records, infection rates, and protocol compliance rates. These systematic approaches to safety ensure optimal outcomes while minimizing risks associated with IV therapy for arthritis.
How Could The Drip IV Infusion Support Arthritis Management Through IV Therapy?
The Drip IV Infusion provides specialized IV therapy services designed to support comprehensive arthritis management through customized treatment protocols. Professional infusion specialists deliver FDA-approved biologic medications and targeted nutrient therapies that address inflammatory processes underlying arthritis symptoms. Treatment plans integrate evidence-based protocols with personalized care approaches to optimize therapeutic outcomes for each patient’s specific arthritis type and severity.
What Services and Customizations Does The Drip IV Infusion Offer for Arthritis Patients?
The Drip IV Infusion offers personalized IV therapy protocols tailored to individual arthritis presentations and treatment goals. Services include administration of biologic DMARDs such as infliximab, tocilizumab, rituximab, and abatacept through controlled infusion protocols. The clinic provides pre-infusion assessments, medication monitoring, and post-treatment observation to ensure safety and efficacy.
Customization options at The Drip IV Infusion encompass:
- Dosage adjustments based on body weight and disease activity
- Infusion rate modifications for patient comfort
- Combination therapy coordination with oral medications
- Flexible scheduling for maintenance infusions
- Symptom-specific nutrient supplementation protocols
The facility maintains CRNI-certified nursing staff trained in arthritis medication administration and infusion reaction management. Treatment rooms feature comfortable seating, entertainment options, and monitoring equipment for extended infusion sessions lasting 2-4 hours.
What Are the Most Important Things to Remember About IV Therapy for Arthritis?
The most important considerations for IV therapy in arthritis management include cost, insurance coverage, and treatment adherence factors. Biologic medications cost between $10,000 and $30,000 per year, with administration costs adding approximately $1,900 per patient annually for IV biologics. Medicare Part D beneficiaries face out-of-pocket expenses of $2,712 to $2,774 for biologic DMARDs before reaching catastrophic coverage.
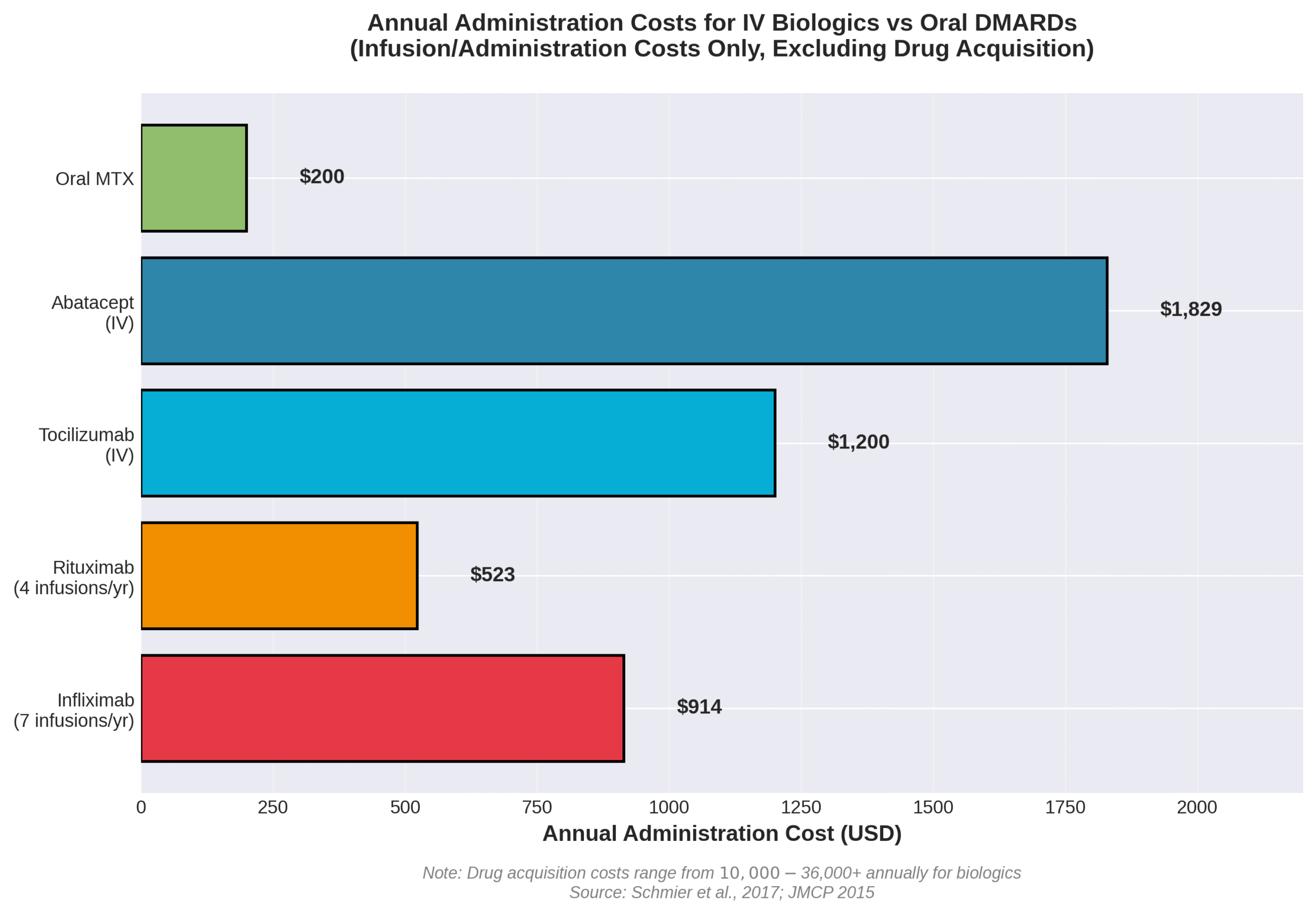
Insurance dynamics significantly impact treatment accessibility. Many arthritis patients switch to IV treatments to reduce out-of-pocket expenses under Medicare, as IV medications fall under Part B medical benefits rather than Part D pharmacy coverage. The Drip IV Infusion assists with insurance verification and prior authorization processes required for most biologic infusions.
Biosimilar adoption rates vary regionally, with 36% of RA patients in Catalonia receiving biosimilars, exceeding the 12% recommendation. However, more than 1 in 8 patients who initiated biosimilar treatment switched back to bio-originator medications like Humira, highlighting the importance of monitoring treatment response and patient preferences when selecting between originator biologics and biosimilars at The Drip IV Infusion.
