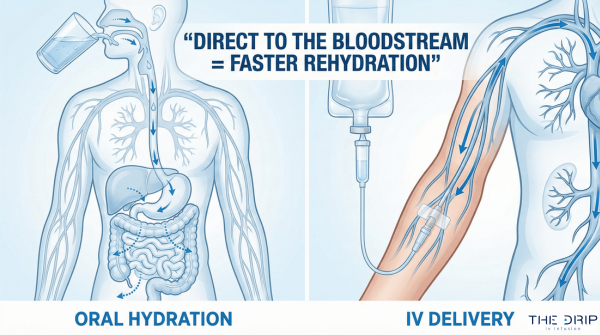
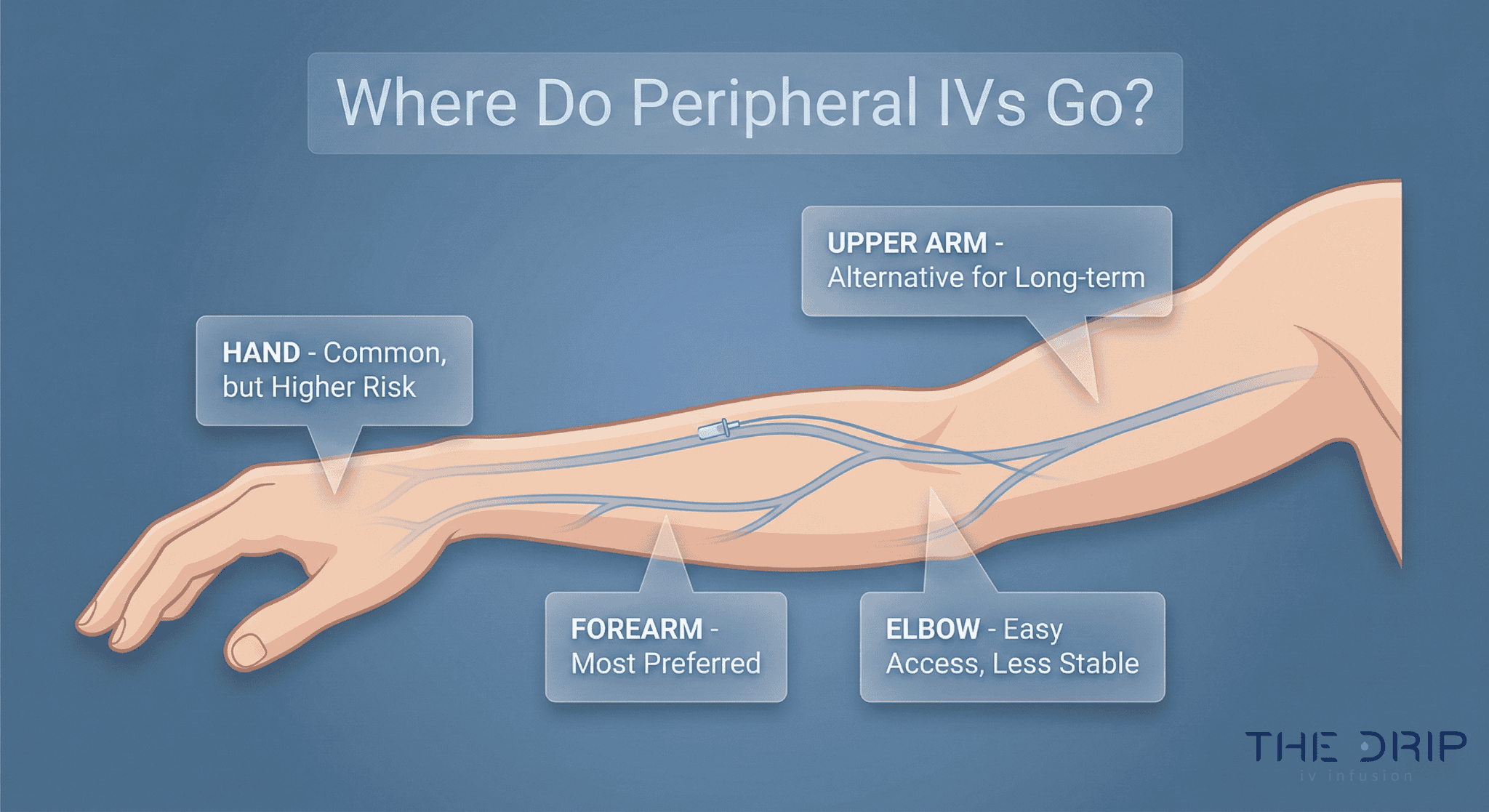
Dehydration usually occurs when the body loses more fluids than it takes in, which may result in impaired metabolic processes. If this condition is severe, oral medications cannot help enough, so IV therapy is recommended for many patients. But why is IV therapy needed, and what is in an IV bag for dehydration?
Read on as this article will explore the composition of IV dehydration bags, the various components used and their application in medical practice.

Source: shutterstock.com / Photo Contributor: EugeneEdge
What Is in an IV Bag for Dehydration?
The IV bag for dehydration combines water, glucose, electrolytes, and sometimes additional additives. The composition of the IV bag may vary depending on the patient’s health conditions and specific needs.
So, what does IV hydration consist of in particular? In the following content, you will find out the IV composition and the main characteristics of its components.
Water
Water is a major component of IV fluids. Its main role is to dissolve the remaining ingredients in the IV bag and deliver them into the bloodstream. Water may also play a key role in regulating body temperature, maintaining cellular functions, and optimizing metabolic processes.
Electrolytes
Are electrolytes included in an IV bag for dehydration? Besides water as the main component, electrolytes are essential for IV hydration therapy. These minerals dissolve in water to form ions necessary for various physiological needs. Electrolytes such as sodium, potassium, chloride, and bicarbonate may often be found in the IV bag.
Sodium: May play an important role in maintaining muscle and nerve function and helping regulate blood pressure.
Potassium: An important electrolyte that may support heart function and maintain the normal balance of fluids in the cell.
Chloride: Works with sodium to maintain fluid balance and osmotic pressure. It may also play an important role in the body’s acid-base balance.
Bicarbonate: May act as a buffer important in maintaining the pH of the blood, preventing it from becoming too alkaline or too acidic.
Glucose
Glucose, an integral part of the IV bag, might be a quick source of energy and help absorb electrolytes. This component may be especially important for patients who have problems consuming food and drinks.
Other additives
Depending on the patient’s specific medical condition, additional ingredients in the IV bag may be included. These components can include vitamins, medicines, and supplements.
Vitamins: Although combining IV fluids with electrolytes may be an ideal choice to combat dehydration, adding vitamins may speed up the recovery process. Certain vitamins, such as B-complex and vitamin C, may provide nutritional support and help maintain the immune system.
Medications: If a specific medical condition is to be helped, medications are included as an integral part of the IV bag. Consultation with your IV nurse is important regarding the type of medication, amount, and frequency of administration.

Source: shutterstock.com / Photo Contributor: Andrey_Popov
Types of IV Fluids for Dehydration
What type of solution is typically in an IV bag for dehydration? IV fluids for dehydration can be classified according to their osmolality, that is, the concentration of dissolved substances in the solution and the osmolality of the blood. In this regard, such fluids are divided into hypertonic, isotonic, and hypotonic.
Isotonic solutions
The main characteristic of isotonic solutions is that they have the same osmotic pressure as blood plasma. This composition allows isotonic solutions to maintain a balanced osmotic pressure with equal amounts of fluids inside and outside the cell.
In addition, these solutions may increase the intravascular volume in several conditions, such as metabolic acidosis, vomiting, diarrhea, etc. In this group of solutions, the most commonly used in medical practice are:
Normal saline (0.9% NaCl)
You may also find the normal saline solution under 0.9NaCL, NS or 9% normal saline. Such a solution easily passes through cell membranes and is generally an isotonic solution.
Due to this feature, normal saline may be used in several conditions, such as vomiting, diarrhea, bleeding, and even shock conditions. In addition, normal saline might also be used when administering blood.
However, this solution may cause excess fluid retention due to its high sodium content. So, caution should be exercised in patients with kidney complications and those with heart disease.
Lactated Ringer’s solution
Lactated Ringer’s is another of the most commonly used isotonic solutions in addition to normal saline. Such a solution is most often found in emergency rooms, ambulances and where emergency cases are treated.
Furthermore, it may be used in patients with extensive blood loss, burns or severe injuries. In some cases, lactated Ringer’s may be used as an alternative to normal saline.
On the other hand, this solution is not advised for patients with kidney complications because it contains potassium. If this condition is not addressed, hyperkalemia may occur.
Hypotonic solutions
The main characteristic of hypotonic solutions is that they move from the bloodstream and pass into the cell through a semipermeable membrane or from the extracellular space to the intracellular space. This solution may help with cellular dehydration by returning the water in the cell to its normal limits.
Also, it may be used in several conditions, such as ketoacidosis. In general, the most commonly used isotonic solutions in medical practice can be:
0.225% NaCl
0.45% NaCl
0.33% NaCl
2.5% dextrose in water
Hypertonic solutions
Unlike hypotonic solutions, where fluids are transferred from extracellular to intracellular spaces, hypertonic solutions have the opposite situation. In such solutions, water is drawn from the cells, instead of being taken in. Because of this feature, hypertonic solutions may be used in patients with low sodium levels.
However, the patient’s health status should be considered before administering such a solution. Hypertonic solutions are not recommended for patients with heart or kidney problems. The most commonly used hypertonic solutions in patients are:
3% NaCl
5% NaCl
5% dextrose in Lactated Ringer’s
5% dextrose in 0.45% NaCl
5% dextrose in 0.9% NaCl
10% dextrose in water
20% dextrose in water
50% dextrose in water
Source: shutterstock.com / Photo Contributor: Numstocker
Conclusion
What is in an IV bag for dehydration? Knowing this is important in planning medical treatment and patient care. So, the IV bag may contain electrolytes, glucose, water, and occasionally other additives.
Moreover, isotonic solutions may be used for general rehydration, while hypertonic and hypotonic solutions may be used in patients with electrolyte imbalances and cellular dehydration problems. Yet, before administering any medication, the patient’s health status, age, metabolism and physical activity should be considered.
So, before undergoing IV treatment, it is recommended that you discuss the benefits and possible risks of IV therapy with your healthcare provider. Take care!