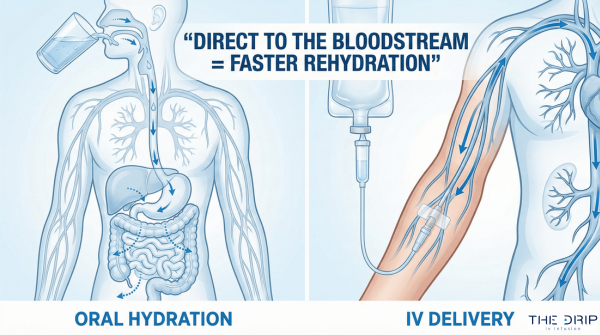
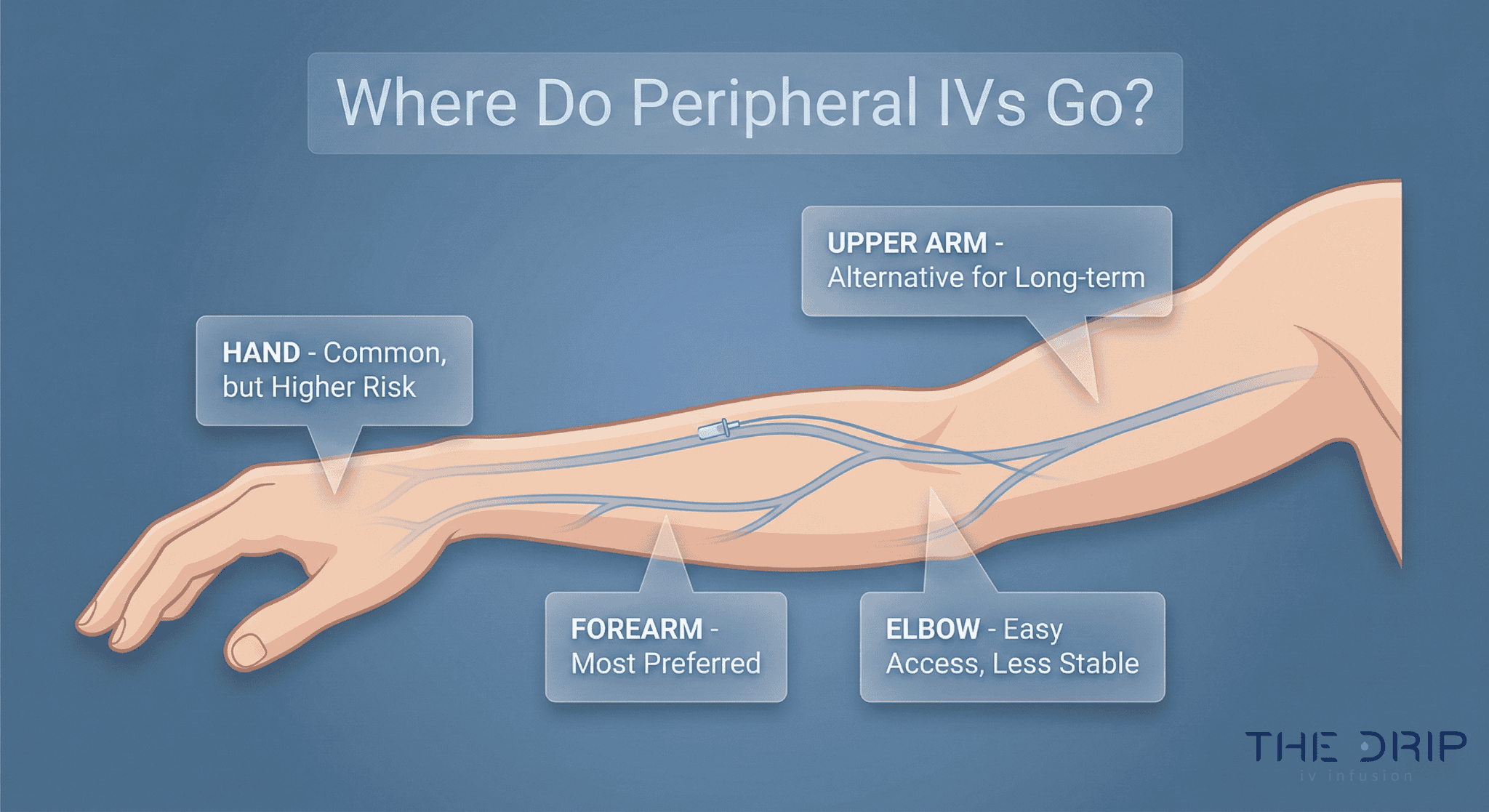
Balancing solutions are crucial for correcting a fluid or electrolyte imbalance in the body. There are three primary types – isotonic, hypotonic, and hypertonic. Knowing when to give isotonic hypotonic and hypertonic solutions is vital for keeping the patient in a stable condition.
Before administering any balancing solution, the medical professional must consider certain factors, such as the patient’s overall condition, fluid levels, and age and weight.
Below, we’ll go over some vital considerations regarding the application and use cases of each type of solution.
Source: shutterstock.com / Photo Contributor: siam.pukkato
When to Give Isotonic Hypotonic and Hypertonic Solutions
Choosing between an isotonic, hypotonic, and hypertonic solution requires careful observation of the patient’s health status and fluid needs. That’s why we at The Drip IV Infusion care for our patient’s health, and our option for mobile IV infusion Arizona helps us observe the person receiving the IV therapy at all times during administration. Here’s a summary of when each type of solution should be used:
Isotonic solutions
Isotonic solutions have nearly the same amount of solutes as the blood. They are given to patients when they lose some fluid due to dehydration or under anesthesia.
Injecting an isotonic solution like normal saline may help replenish the body and return its fluid balance. It is given as extracellular fluid to avoid disrupting the normal functioning of the cells.
Common isotonic solutions include:
0.9% NaCl (normal saline) – A solution with the same sodium concentration found in the extracellular fluid. It can be administered for low blood pressure (hypotension).
Lactated Ringer’s solution – Contains electrolytes (sodium, potassium, calcium, and lactate) similar to extracellular fluid. It is used to replenish fluids and electrolytes in the body and sometimes as an alkalizing agent to balance the pH levels.
5% dextrose in water (D5W) – Contains glucose and is used to provide carbohydrates and fluid balance to the body. It’s used to potentially treat low blood sugar, insulin shock, or dehydration.
Use cases
Here’s when an isotonic solution may be used:
Dehydration – isotonic solutions are most commonly used to treat dehydration caused by vomiting, diarrhea, excessive sweating, or inadequate fluid intake. They may help restore fluid balance in the body without causing a shift of water into or out of cells.
Intravenous (IV) fluid replacement – when undergoing surgery, the patient is susceptible to significant fluid loss. An isotonic solution is administered through an IV to keep the fluid levels balanced and prevent complications. It can also help maintain blood volume and electrolyte levels.
Blood transfusions – isotonic, or more specifically Ringer’s solution, is often used during blood transfusions to dilute blood products (red blood cells, platelets, plasma) and prevent adverse reactions. They reduce the stress on the red blood cells, preventing them from swelling or shrinking. Isotonic solutions also slow the introduction of the blood product into the system, allowing it to gradually adjust to the change.
Shock treatment – during hypovolemic shock, severe fluid loss leads to improper heart functioning, preventing the heart from pumping enough blood to the entire body. In such cases, an isotonic solution may be used to expand blood volume and restore tissue perfusion.
Source: shutterstock.com / Photo Contributor: AlteredR
Hypotonic solutions
Hypertonic solutions have fewer solutes than the amount of solutes in the blood. They are administered to hydrate the cells. A hypotonic solution is used in specific medical situations where it’s necessary to expand the intracellular fluid volume.
Common hypotonic solutions include:
0.45% NaCl (half-normal saline) – Contains 0.45% sodium chloride (salt) and is hypotonic compared to normal body fluids. It is used to treat conditions that lead to cellular dehydration.
0.33% NaCl – This solution has an even lower concentration of salt than the previous one and is considered more hypotonic. It may be used in situations where a milder hypotonic effect is needed.
Dextrose 2.5% in water (D2.5W) – While it is initially isotonic, D2.5W becomes hypotonic once the dextrose is metabolized. Its purpose remains the same, except that it needs regular monitoring to avoid adverse effects like cellular swelling – a possible reaction from the shift in tonicity.
Use cases
Here’s when a hypotonic solution is commonly used:
Cellular dehydration – this is the primary use of hypotonic solutions. There are many conditions that can lead to cellular dehydration as a result of a loss of intracellular fluid. One example is diabetic ketoacidosis, which can initially be treated with an isotonic solution but may require hypotonic solution administration in more severe cases. Another example is hypernatremia, which is a result of elevated sodium levels.
Hypertonic solutions
Hypertonic solutions have more solutes than the amount of solutes in the blood. Their use is opposite from that of hypotonic solutions – instead of moving fluid into the cells, they are used to drive out excess fluid.
Common hypertonic solutions include:
3% and 5% NaCl (3% and 5% saline) – These solutions have a higher concentration of sodium chloride (salt) compared to normal body fluids. Thus, they can be used to treat conditions that lead to saline deficit, like hyponatremia and cerebral edema.
Dextrose 10% in water (D10W) – Hypertonic solution with a high concentration of dextrose used to provide extra water and carbohydrates for more energy. It can be used to treat hypoglycemia and as part of total parenteral nutrition – when the patient receives nutrients exclusively through an IV.
Use cases
Here’s when a hypertonic solution may be used:
Severe hyponatremia – Hypertonic solutions are used in cases where the sodium levels in the blood are dangerously low. In this case, only a severe form of hyponatremia should be treated with a hypertonic solution to avoid administering too much saline.
Cerebral edema – Hypertonic solutions may help reduce brain swelling in traumatic brain injuries, cerebral hemorrhage, or other neurological conditions.
Important Factors to Consider
Going simply off the use cases of each type of IV solution is not enough to determine which one’s right for the patient. Here are some factors that should be considered when deciding when to give isotonic hypotonic and hypertonic solutions:
Age and weight – Fluid needs differ among age and weight demographics. Careful consideration can prevent underdosing or overdosing.
Fluid status – Underhydrated vs. overhydrated patients require different IV solutions.
Electrolyte imbalance – Knowing the patient has an electrolyte imbalance is not enough. Every condition with an electrolyte imbalance requires a unique approach based on the overall fluid status.
Underlying medical conditions – Patients with chronic conditions may have unique fluid needs that should be addressed accordingly.

Source: shutterstock.com / Photo Contributor: Rabbitti
Conclusion
When to give isotonic hypotonic and hypertonic solutions? That depends on the patient’s condition, fluid status, age and weight, and possible underlying medical conditions.
Isotonic solutions are used in fluid maintenance therapy and are generally administered to aid hydration, especially post-op. They may also be given during blood transfusions to dilute any blood products.
Hypotonic solutions may help with cellular dehydration by restoring the cell’s normal volume.
Finally, hypertonic solutions are administered in the case of extra-low sodium levels. They drive out excess fluid from the cells to potentially optimize the fluid levels.